Battery Of A Voltaic Cell . Though a variety of electrochemical cells exist, batteries generally consist of at least one voltaic cell. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. How to use a redox reaction to construct a galvanic/voltaic cell to produce a flow of current. Shows the flow of electrons and. A simple voltaic cell uses zinc and copper electrodes in diluted sulfuric acid to generate electricity, illustrating the basic battery working principle. Voltaic cells are also sometimes referred. Voltaic cells produce electricity, while electrolytic cells use a power source to drive a reaction forward.
from chem.libretexts.org
Voltaic cells produce electricity, while electrolytic cells use a power source to drive a reaction forward. How to use a redox reaction to construct a galvanic/voltaic cell to produce a flow of current. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. A simple voltaic cell uses zinc and copper electrodes in diluted sulfuric acid to generate electricity, illustrating the basic battery working principle. Voltaic cells are also sometimes referred. Shows the flow of electrons and. Though a variety of electrochemical cells exist, batteries generally consist of at least one voltaic cell.
20.3 Voltaic Cells Chemistry LibreTexts
Battery Of A Voltaic Cell How to use a redox reaction to construct a galvanic/voltaic cell to produce a flow of current. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. How to use a redox reaction to construct a galvanic/voltaic cell to produce a flow of current. Shows the flow of electrons and. Though a variety of electrochemical cells exist, batteries generally consist of at least one voltaic cell. Voltaic cells produce electricity, while electrolytic cells use a power source to drive a reaction forward. Voltaic cells are also sometimes referred. A simple voltaic cell uses zinc and copper electrodes in diluted sulfuric acid to generate electricity, illustrating the basic battery working principle.
From www.dreamstime.com
Battery current stock illustration. Illustration of physics 212689705 Battery Of A Voltaic Cell Shows the flow of electrons and. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. Voltaic cells produce electricity, while electrolytic cells use a power source to drive a reaction forward. Voltaic cells are also sometimes referred. A simple voltaic cell uses zinc and copper electrodes in diluted. Battery Of A Voltaic Cell.
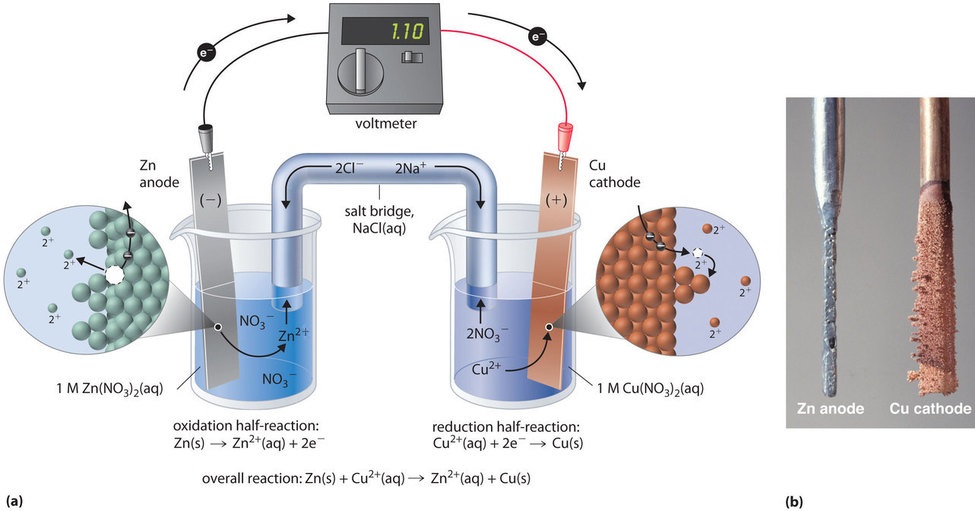
From 2012books.lardbucket.org
Describing Electrochemical Cells Battery Of A Voltaic Cell A simple voltaic cell uses zinc and copper electrodes in diluted sulfuric acid to generate electricity, illustrating the basic battery working principle. How to use a redox reaction to construct a galvanic/voltaic cell to produce a flow of current. Voltaic cells are also sometimes referred. Though a variety of electrochemical cells exist, batteries generally consist of at least one voltaic. Battery Of A Voltaic Cell.
From overallscience.com
Voltaic Cell, Its Construction and Defects Overall Science Battery Of A Voltaic Cell A simple voltaic cell uses zinc and copper electrodes in diluted sulfuric acid to generate electricity, illustrating the basic battery working principle. Voltaic cells are also sometimes referred. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. Voltaic cells produce electricity, while electrolytic cells use a power source. Battery Of A Voltaic Cell.
From www.slideserve.com
PPT Voltaic Cells PowerPoint Presentation, free download ID3061019 Battery Of A Voltaic Cell A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. Though a variety of electrochemical cells exist, batteries generally consist of at least one voltaic cell. How to use a redox reaction to construct a galvanic/voltaic cell to produce a flow of current. A simple voltaic cell uses zinc. Battery Of A Voltaic Cell.
From etc.usf.edu
Voltaic Cell ClipArt ETC Battery Of A Voltaic Cell How to use a redox reaction to construct a galvanic/voltaic cell to produce a flow of current. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. A simple voltaic cell uses zinc and copper electrodes in diluted sulfuric acid to generate electricity, illustrating the basic battery working principle.. Battery Of A Voltaic Cell.
From digital.sciencehistory.org
Reproduction of Voltaic Pile Science History Institute Digital Battery Of A Voltaic Cell Though a variety of electrochemical cells exist, batteries generally consist of at least one voltaic cell. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. Voltaic cells produce electricity, while electrolytic cells use a power source to drive a reaction forward. Voltaic cells are also sometimes referred. Shows. Battery Of A Voltaic Cell.
From favpng.com
Voltaic Pile Galvanic Cell Electric Battery Electricity Invention, PNG Battery Of A Voltaic Cell How to use a redox reaction to construct a galvanic/voltaic cell to produce a flow of current. Though a variety of electrochemical cells exist, batteries generally consist of at least one voltaic cell. A simple voltaic cell uses zinc and copper electrodes in diluted sulfuric acid to generate electricity, illustrating the basic battery working principle. A voltaic cell (also known. Battery Of A Voltaic Cell.
From peoi.org
Chapter 14 Section C Applications of Redox Reactions Voltaic Cells Battery Of A Voltaic Cell Shows the flow of electrons and. Voltaic cells produce electricity, while electrolytic cells use a power source to drive a reaction forward. Though a variety of electrochemical cells exist, batteries generally consist of at least one voltaic cell. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. A. Battery Of A Voltaic Cell.
From stock.adobe.com
Galvanic voltaic cell infographic diagram battery part structure Battery Of A Voltaic Cell Shows the flow of electrons and. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. Though a variety of electrochemical cells exist, batteries generally consist of at least one voltaic cell. Voltaic cells are also sometimes referred. Voltaic cells produce electricity, while electrolytic cells use a power source. Battery Of A Voltaic Cell.
From chem.libretexts.org
20.3 Voltaic Cells Chemistry LibreTexts Battery Of A Voltaic Cell Voltaic cells produce electricity, while electrolytic cells use a power source to drive a reaction forward. Shows the flow of electrons and. How to use a redox reaction to construct a galvanic/voltaic cell to produce a flow of current. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity.. Battery Of A Voltaic Cell.
From www.youtube.com
Electrochemistry Galvanic/Voltaic Cell Battery Made Super Simple! MCAT Battery Of A Voltaic Cell Though a variety of electrochemical cells exist, batteries generally consist of at least one voltaic cell. Voltaic cells produce electricity, while electrolytic cells use a power source to drive a reaction forward. How to use a redox reaction to construct a galvanic/voltaic cell to produce a flow of current. A simple voltaic cell uses zinc and copper electrodes in diluted. Battery Of A Voltaic Cell.
From www.pinterest.com
Alessandro Volta Voltaic Pile Energy storage, Inventions, Voltaic pile Battery Of A Voltaic Cell Though a variety of electrochemical cells exist, batteries generally consist of at least one voltaic cell. Voltaic cells produce electricity, while electrolytic cells use a power source to drive a reaction forward. Voltaic cells are also sometimes referred. A simple voltaic cell uses zinc and copper electrodes in diluted sulfuric acid to generate electricity, illustrating the basic battery working principle.. Battery Of A Voltaic Cell.
From www.differencebetween.com
What is the Difference Between Voltaic Cell and Electrolytic Cell Battery Of A Voltaic Cell A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. Though a variety of electrochemical cells exist, batteries generally consist of at least one voltaic cell. A simple voltaic cell uses zinc and copper electrodes in diluted sulfuric acid to generate electricity, illustrating the basic battery working principle. Voltaic. Battery Of A Voltaic Cell.
From www.slideserve.com
PPT Electrochemical Cells (voltaic cells) PowerPoint Presentation Battery Of A Voltaic Cell Voltaic cells produce electricity, while electrolytic cells use a power source to drive a reaction forward. Though a variety of electrochemical cells exist, batteries generally consist of at least one voltaic cell. How to use a redox reaction to construct a galvanic/voltaic cell to produce a flow of current. A simple voltaic cell uses zinc and copper electrodes in diluted. Battery Of A Voltaic Cell.
From www.electricity-magnetism.org
Voltage of Electric Batteries Cell Voltage Electricity Battery Of A Voltaic Cell A simple voltaic cell uses zinc and copper electrodes in diluted sulfuric acid to generate electricity, illustrating the basic battery working principle. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. Shows the flow of electrons and. How to use a redox reaction to construct a galvanic/voltaic cell. Battery Of A Voltaic Cell.
From www.britannica.com
John Frederic Daniell electrolytic cell, voltaic pile, battery Battery Of A Voltaic Cell Shows the flow of electrons and. Though a variety of electrochemical cells exist, batteries generally consist of at least one voltaic cell. How to use a redox reaction to construct a galvanic/voltaic cell to produce a flow of current. Voltaic cells produce electricity, while electrolytic cells use a power source to drive a reaction forward. A voltaic cell (also known. Battery Of A Voltaic Cell.
From www.pinterest.com
Kognity Chemistry classroom, Electrochemistry, Chemistry lessons Battery Of A Voltaic Cell Voltaic cells produce electricity, while electrolytic cells use a power source to drive a reaction forward. Shows the flow of electrons and. A simple voltaic cell uses zinc and copper electrodes in diluted sulfuric acid to generate electricity, illustrating the basic battery working principle. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous. Battery Of A Voltaic Cell.
From www.indiamart.com
Lead Acid Battery/ Voltaic Cell Model at Rs 1250 in Indore ID Battery Of A Voltaic Cell A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. How to use a redox reaction to construct a galvanic/voltaic cell to produce a flow of current. Voltaic cells are also sometimes referred. Though a variety of electrochemical cells exist, batteries generally consist of at least one voltaic cell.. Battery Of A Voltaic Cell.